The aim of our research is to understand how complex traits vary and evolve. Many traits of interest differ between individuals as a result of a complicated interplay between genetic differences, environmental differences, and random chance.
A major feature of many complex traits is their robustness (Masel & Siegal 2009). That is, the regulatory networks that produce these traits suppress the effects of both genetic and nongenetic perturbations. Our lab uses a variety of approaches to understand the causes and evolutionary consequences of this robustness. We also study cases where variability (or the lack of robustness) appears to be advantageous. Such cases include so-called bet-hedging mechanisms, whereby a population maximizes its long-term success in an uncertain environment by maintaining subpopulations that thrive under different conditions (Levy et al. 2012). Our studies of robustness have also led us to study genetic changes and their effects more generally, including the fitness effects of new mutations (Plavskin et al. 2024) and how genetic interactions determine trait outcomes (Buzby et al. 2025). Our work primarily focuses on the budding yeast, Saccharomyces cerevisiae, but has also involved another major model organism, the fly Drosophila melanogaster, as well as theoretical work involving computational simulations and analysis of human data from genome-wide association studies.
Robustness
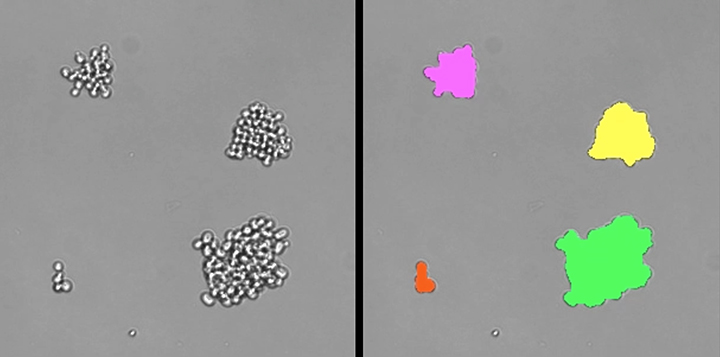
One major experimental focus in our lab is on directly identifying and characterizing genes that contribute to robustness of many traits. We have screened the genome of S. cerevisiae for genes whose deletion increases the variation in the morphologies of individual, genetically identical cells (i.e., the genes normally contribute to robustness against chance fluctuations in the external or internal cellular environment). Hundreds of nonessential yeast genes increase morphological variation when deleted, and these genes tend to be highly connected in cellular networks (Levy & Siegal 2008). An even greater proportion of essential genes contribute to robustness against environmental fluctuations (Bauer et al. 2015). We have also tested whether the same mechanisms that buffer environmental fluctuations also buffer the effects of mutations. A longstanding hypothesis in the field has been that the two types of buffering should be linked mechanistically, but we have refuted this connection in the case of the chromatin protein H2A.Z (Richardson et al. 2013) and the molecular chaperone Hsp90 (Geiler-Samerotte et al. 2016). Indeed, our work supports the view that selection against unbuffered alleles leaves the false impression that proteins such as Hsp90 preferentially buffer the effects of mutations (Geiler-Samerotte et al. 2016).
Bet Hedging
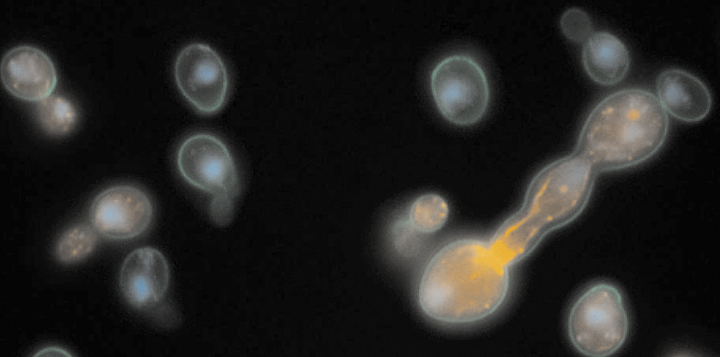
A second major experimental focus is on our discovery of a putative bet-hedging system in S. cerevisiae using a high-throughput, microscopy-based growth assay that we developed (Levy et al. 2012; Plavskin et al. 2021; Sartori et al. 2021). Individual yeast cells show a large amount of variation in growth rate, and these differences are transiently heritable. Slower-growing cells are naturally outcompeted by faster-growing cells when conditions are benign, but are better able to survive acute stress. Different strains of yeast differ in their growth-rate distributions, suggesting that ecological pressures might shape bet-hedging strategies in nature (Ziv et al. 2013; Ziv et al. 2017). We are currently working on trying to understand the molecular mechanism underlying heterogeneity in growth rate and stress resistance (Li et al. 2018), as well as its divergence between strains and species (Ziv et al. 2017).
Genetic Changes and Their Effects
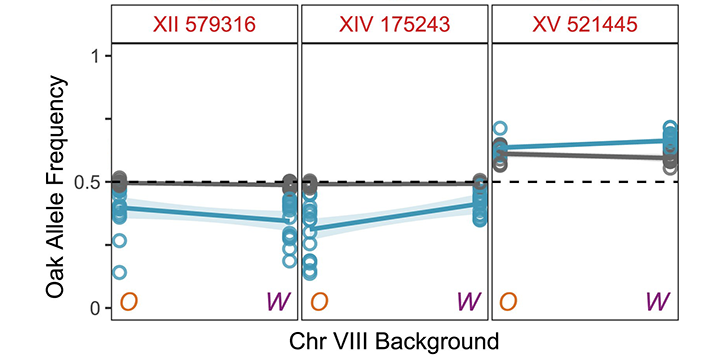
A third major experimental focus is on how mutations enter populations and impact complex traits. We have studied how the mismatch repair system prevents mutations in simple sequence repeats (SSRs) such as repeated dinucleotide and trinucleotide sequences (Plavskin et al. 2023), and we have shown that SSR mutations frequently alter the growth rates of yeast cells, making them an important source of fitness variation (Plavskin et al. 2024). We have also developed a powerful new method for identifying genetic interactions that contribute to complex-trait variation (Buzby et al. 2025). We engineer yeast strains to enable crosses where one parent’s chromosome is fixed while the rest of the chromosomes segregate. These crosses allow us to use the power of bulk-segregant analysis to identify quantitative trait loci (QTL) whose effects depend on alleles on the fixed parental chromosome.